|
How Lead Acid
Batteries Work
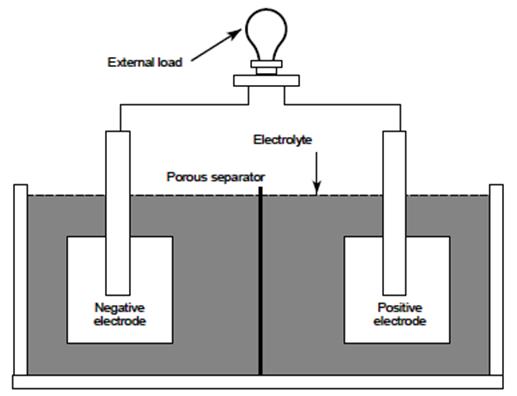
The negative
electrode supplies electrons to the external circuit (or load)
during discharge. In a fully charged lead-acid storage battery the negative
electrode is composed of sponge lead (Pb). The positive electrode
accepts electrons from the load during discharge. In a fully charged
lead-acid battery the positive electrode is composed of lead dioxide (PbO2).
It should be noted that the electrodes in a battery must be of dissimilar
materials or the cell will not be able to develop an electrical potential
and conduct electrical current. The electrolyte completes the
internal circuit in the battery by supplying ions to the positive and
negative electrodes. Diluted sulfuric
acid (H2SO4) is the electrolyte in lead-acid
batteries. In a fully charged lead-acid battery, the electrolyte is
approximately 37.52% sulfuric acid and 62.48% water, or 1.285 specific
gravity.
The separator
is used to electrically isolate the positive and negative electrodes. If
the electrodes are allowed to come in contact, the cell will short-circuit
and become useless because both electrodes would be at the same
potential. The type of separator
used varies by cell type. Materials used as separators
must allow ion transfer between the electrolyte and electrodes. Many
separators are made of a porous plastic or glass fiber material. The above
components are housed in a container commonly called a jar to
form a cell.
Cells and batteries
may be connected in series, parallel, or combinations of both. Cells or
batteries connected in series have the positive terminal of one cell or
battery connected to the negative terminal of another cell or battery. This
has the effect of increasing the overall voltage but the overall capacity
remains the same. For example, a 12V lead-acid automobile battery contains
6 cells connected in series with each cell having a potential difference of
about 2V.
 Back to top Back to top
|